Your Liquid Nitrogen Safety Resource Center


The use of liquid nitrogen (LIN) has grown in popularity for use in many end user applications, including food and beverage applications, cryotherapies, and creating cloud or smoky effects. Liquid nitrogen is extremely cold (–320 °F / –196 °C) and rapidly converts into nitrogen gas at room temperature. In recent years, there have been incidents that have resulted in serious injuries and deaths associated with the use of liquid nitrogen.
Anyone who handles liquid nitrogen should be aware of its unique properties and hazards. These include extremely cold temperature, potential to create an oxygen-deficient atmosphere, and rapid conversion from a liquid to a gas. Because of these and other considerations, it is very important that users read and follow the instructions for the equipment being used and safety precautions provided by their liquid nitrogen supplier.
These safety posters, provided by the Compressed Gas Association, provide basic safety information for the safe use of liquid nitrogen in food and beverage service environments and in cryotherapy. Download your free copies today!
To learn more about liquid nitrogen, see the section, “Product Information: Nitrogen” at the bottom of this page.
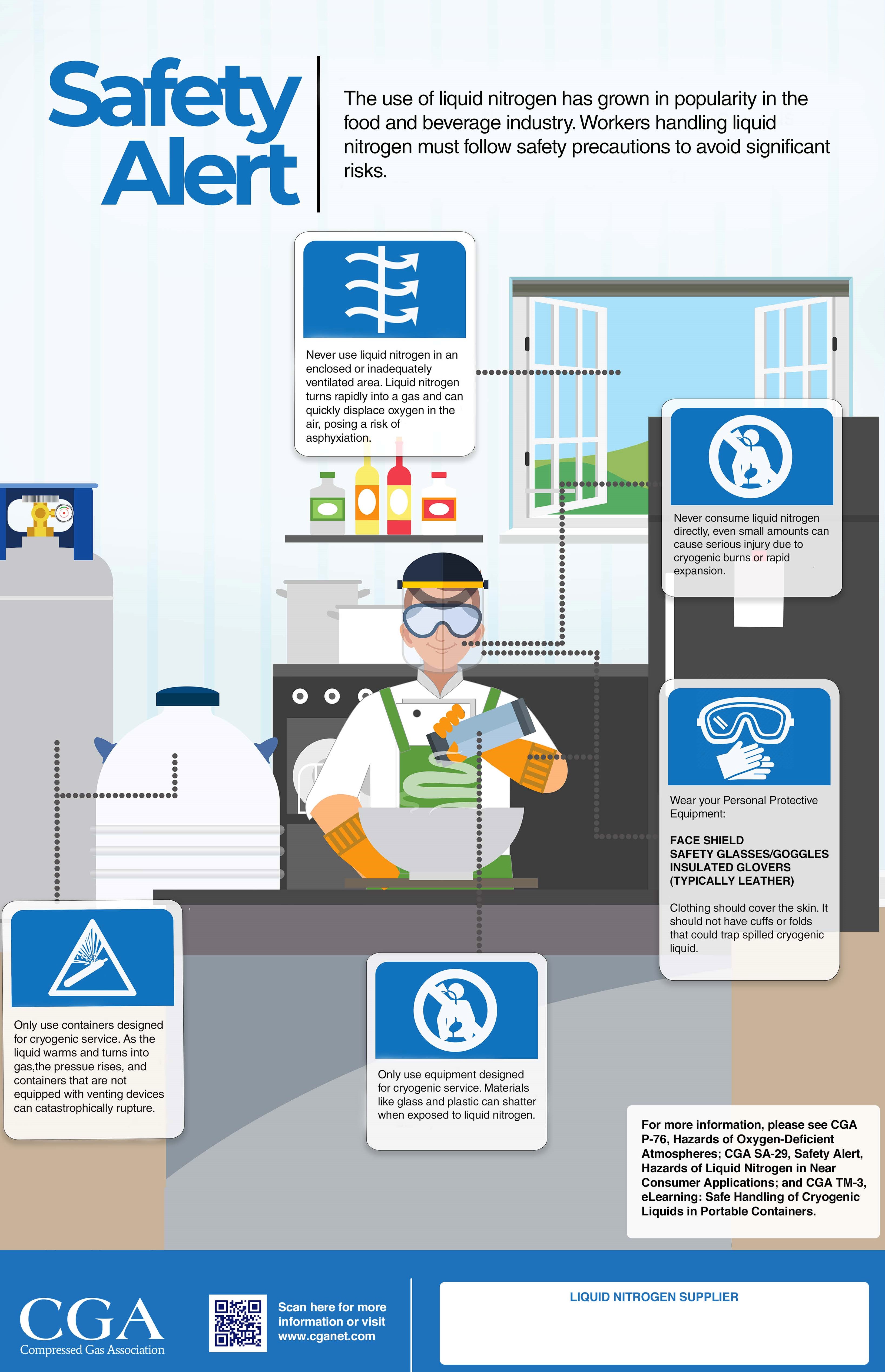
Liquid Nitrogen Safety Reminders
- Wear appropriate personal protective equipment (PPE)
- Never consume liquid nitrogen directly
- Do not lower your head into a liquid nitrogen vapor cloud
- Use liquid nitrogen in well-ventilated areas
- Only use containers and equipment designed for cryogenic service
- Never trap liquid nitrogen in a container, tubing, or piping
- Read and understand safety information prior to using liquid nitrogen
Poster Downloads
CGA offers liquid nitrogen safety posters in multiple designs and formats as a free safety resource. It is important to note that these posters are not a substitute for reading and following codes and regulations, industry standards, equipment manufacturer, and supplier instructions. Download your free liquid nitrogen safety poster today!
NOTE – Use self-print files for printing at your home or office, and full bleed files for professional printing.

Poster 1
Poster 2

Poster 3
Additional Resources
- CGA P-76, Hazards of Oxygen-Deficient Atmospheres
- CGA SA-29, Safety Alert, Hazards of Liquid Nitrogen in Near-Consumer Applications
- CGA TM-3, eLearning: Safe Handling of Cryogenic Liquids in Portable Containers
- CGA P-12, Safe Handling of Cryogenic Liquids
- CGA P-9, The Inert Gases: Argon, Nitrogen, and Helium
Product Information: Nitrogen
Nitrogen makes up more than 75% of the atmosphere. It is a colorless, odorless, tasteless, nontoxic, almost totally inert gas, and it is colorless as a liquid. Nitrogen is nonflammable, will not support combustion, and is not life supporting. It combines with some of the more active metals such as lithium and magnesium to form nitrides, and at high temperatures it will also combine with hydrogen, oxygen, and other elements.
Liquid nitrogen is extremely cold (–320 °F / –196 °C) and can quickly burn or freeze body tissue. It rapidly turns into a gas and expands rapidly at room temperature. For example, one cubic foot of liquid can expand into 700 cubic feet of gas.
For basic safety information on the handling of compressed gas containers, refer to CGA P-1, Safe Handling of Compressed Gases in Containers. In addition, all of the precautions necessary for the handling of any nonflammable gas or cryogenic liquid must be taken; see your liquid nitrogen supplier’s safety data sheets and CGA P-9, The Inert Gases, Argon, Nitrogen, and Helium. Nitrogen can act as a simple asphyxiant by diluting the concentration of oxygen in air below levels necessary to support life; see CGA P-76, Oxygen‑Deficient Atmospheres, for safety recommendations.
Liquid and gaseous systems should be designed and installed only under the direction of personnel thoroughly familiar with liquid and gaseous nitrogen equipment, and in compliance with state, territorial, provincial, and local requirements.
Disposal of gaseous or liquid nitrogen should only be done by trained, competent personnel. This must be carried out in a responsible manner to ensure that no hazardous conditions are created, all regulatory requirements are met and there is no risk of damage to the environment. See CGA P-63, Disposal of Gases, for additional information.
In the case of a nitrogen leak or liquid nitrogen spill:
- ventilate adjacent enclosed areas to prevent the formation of oxygen-deficient atmospheres;
- evacuate the area;
- DO NOT enter areas where the oxygen concentration is below 19.5% unless provided with a self-contained breathing apparatus (SCBA) or air-line respirator; and
- call your liquid nitrogen supplier immediately for assistance.
- Avoid contact of the skin with liquid nitrogen or its cold boil-off gas. Flush liquid nitrogen spills with water to accelerate evaporation.
Inhalation. Nitrogen is generally inert and can cause asphyxiation due to displacement of oxygen in the atmosphere. Rescuers wearing an SCBA or air-line respirators should move the affected person from the hazardous exposure to fresh air at once. If supplemental oxygen is available, administer by nasal cannula or mask. Perform artificial respiration if the person is not breathing. Persons who have been unconscious should be taken to a hospital for evaluation and care.
Skin contact. In case of frostbite from exposure to liquid nitrogen, the frostbitten part should be placed in warm water, 100 °F to 105 °F (37.8 °C to 40.6 °C). If warm water is not available, or it is impractical to use, wrap the affected part gently in blankets. Let circulation re-establish itself naturally. Encourage the victim to exercise the affected part while it is being warmed. Consult a physician.
Nitrogen is nontoxic and largely inert. It can act as a simple asphyxiant by diluting the concentration of oxygen in air below levels necessary to support life. Inhalation of nitrogen in excessive concentrations can result in dizziness, nausea, vomiting, loss of consciousness, and death. Death can result from errors in judgment, confusion, or loss of consciousness, which prevents self-rescue. At low oxygen concentrations, unconsciousness and death can occur in seconds without warning.
Gaseous nitrogen must be handled with all the precautions necessary for safety with any nonflammable, nontoxic compressed gas. All precautions necessary for the safe handling of any gas liquefied at very low temperatures must be observed with liquid nitrogen. Extensive tissue damage or burns can result from exposure to liquid nitrogen or cold nitrogen vapors. See CGA P-12, Safe Handling of Cryogenic Liquids, for further information.
Physical constants can be found in the CGA Handbook page for Nitrogen